The Bohr-Einstein Debate
The year is 1905. Newly graduated with a Ph.D. in physics, Albert Einstein publishes the paper Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichttspunkt (“On a Heuristic Viewpoint Concerning the Production and Transformation of Light”).

A narration of the "Debate of the Century"
The year is 1905. Newly graduated with a Ph.D. in physics, Albert Einstein publishes the paper Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichttspunkt (“On a Heuristic Viewpoint Concerning the Production and Transformation of Light”). In it, he proposes a revision to one of the fundamental laws of physics to account for the behavior of light as both a particle and a wave, work for which he would later be awarded the Nobel Prize (1921). Eight years later in 1913, in the paper On the Constitution of Atoms and Molecules, Part II Systems Containing Only a Single Nucleus, Danish physicist Niels Bohr adapts Ernest Rutherford’s 1911 model of the atom to Max Planck’s quantum theory to introduce a new model of the atom — the Bohr model, both earning himself his own Nobel (1922), as well as setting the stage for a coming quantum revolution in physics.
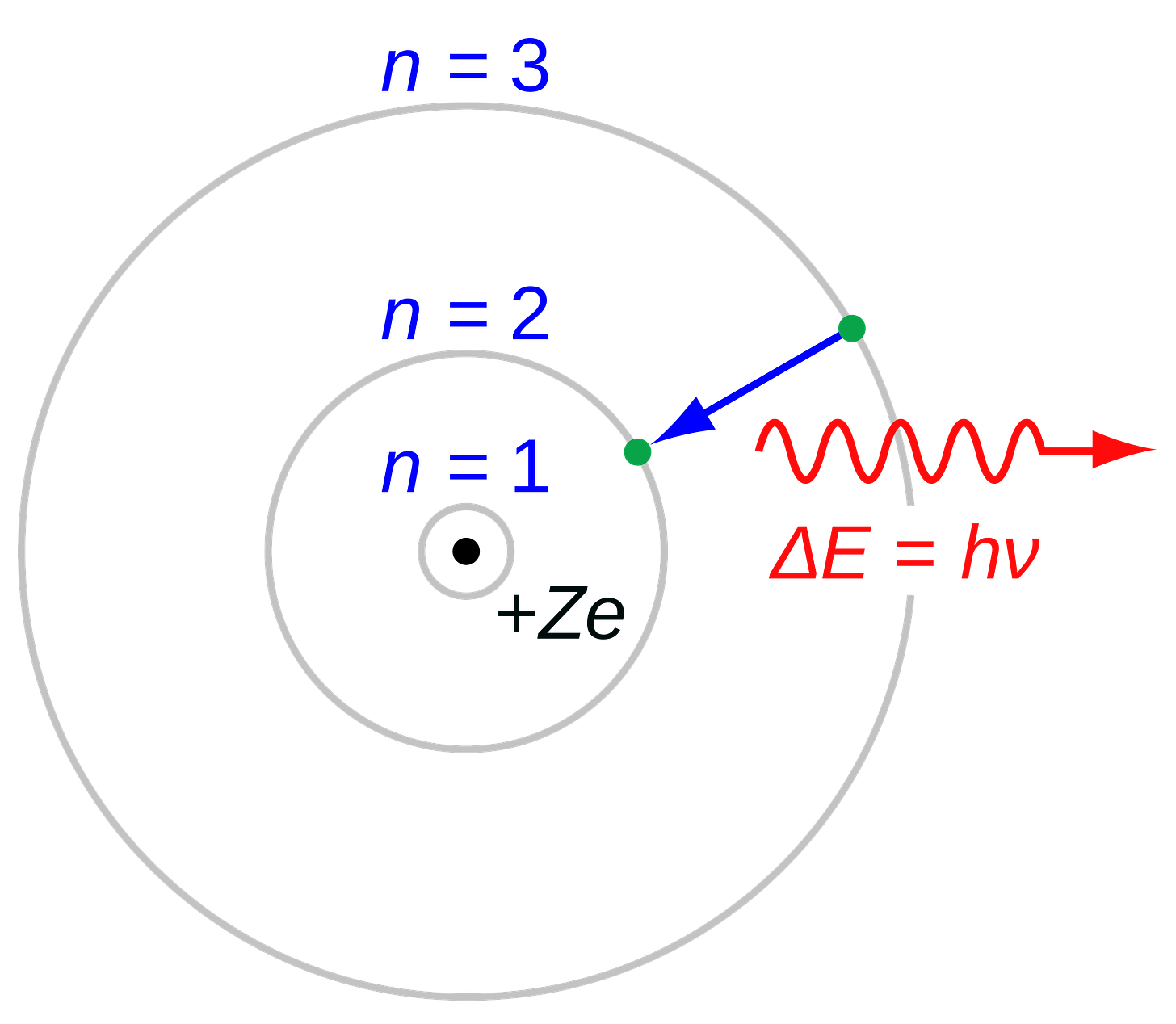
Fast forward 12 years. Building on the work of both Einstein and Bohr, Werner Heisenberg introduces matrix equations which remove the foundational elements of space and time from the then increasingly popular quantummechanical model of physics. Building on this work, Max Born in the following year proposes that mechanics are most effectively understood not as causal links but as actions resulting from probability, not distinct causation. With Heisenberg’s solution of the Schrödinger equation for a scattering problem the following year, the now well-known Heisenberg uncertainty principle is introduced, leading Born and Heisenberg to declare that quantum mechanics was now “complete and irrevocable”.
In less than 25 years, starting with Planck’s 1900 discovery of the black body radiation law, to Einstein’s discovery of the photon, to Bohr’s redefinition of the model of the atom, to Heisenberg and Born’s refinement of quantum mechanics, physics in the first quarter of the twentieth century went from fully deterministic to seemingly indeterminate.
The Fifth Solvay International Conference (1927)
The Bohr-Einstein debate is generally considered to have begun during the Fifth Solvay International Conference on Photons and Electrons. The conference was held in October 1927 in Brussels, Belgium. Continuing on since the successful inaugural conference of 1911, the Solvay gatherings are devoted to outstanding preeminent open problems in physics, and occur approximately every three years. From 1913 to 1961, every gathering revolved around open problems in quantum theory. Chaired by Hendrik Lorentz in 1927, the official topic of the conference was “photons and electrons”. In practice, the 1927 conference revolved around the growing dispute between two then nascent schools of physics: those fascinated and enthralled by the new developments in quantum theory, and those still clinging to the superseded deterministic paradigm. The former was lead by Niels Bohr and the latter by Albert Einstein.

The Copenhagen Interpretation
The open problem during the 1927 Solvay conference was how physicists should interpret the recent results of physicists Werner Heisenberg and Max Born, the now so-called “interpretation question” of quantum mechanics. Born and Heisenberg, fervent in their view, promoted the following (simplified) view:
“Physical systems do not have definite properties prior to being measured. Quantum mechanics can only predict the probability distribution of given measurements’ possible results.”
This because, as the view goes, the act of measurement affects the system being measured. This causes the set of probabilities to reduce to only one of the possible values immediately after the measurement — the so-called wave function collapse. In other words, prior to the measurement of (for instance) the position of an electron, its location is best described by a probability distribution (a wave function). In the act of measuring the position of the electron, the device measuring or observing the electron influences the probability distribution. After the measurement, due to the influence of the observer, the position of the electron is now best defined by a single value (e.g. a Cartesian coordinate).
Definition
“Despite an extensive literature which refers to, discusses, and criticizes the Copenhagen interpretation of quantum mechanics, nowhere does there seem to be any concise statement which defines the full Copenhagen interpretation.”
Despite statements such as the one given above by John G. Cramer in 1986 and many more both before and after it, for the purposes of this article we can colloquially state the Copenhagen interpretation as
The Copenhagen Interpretation of Quantum Mechanics. Physical systems generally do not have definite properties prior to being measured, and quantum mechanics can only predict the probability distribution of a given measurement's possible results. The act of measurement affects the system, causing the set of probabilities to reduce to only one of the possible values immediately after the measurement.
More specifically, we can define it as synonymous with a sum of the concepts of indeterminism, Bohr’s correspondence principle, Born’s statistical interpretation of the wave function and Bohr’s complementarity interpretation of certain atomic phenomena. The term itself stems from Heisenberg who worked as an assistant under Bohr at his institute in Copenhagen while he formulated his uncertainty principle, and can been traced to Heisenberg’s 1930 textbook The Physical Principles of the Quantum Theory in which he states that
"On the whole, the book contains nothing that is not to be found in previous publications, particularly in the investigations of Bohr. The purpose of the book seems to me to be fulfilled if it contributes somewhat to the diffusion of that Copenhagen spirit of quantum theory if I may so express myself, which has directed the entire development of modern atomic physics."
Excerpt, “The Physical Principles of the Quantum Theory” by Werner Heisenberg (1930)
History
In the years from 1925 up until the conference in 1927, the quantum revolution that had been taking place had been propelled mainly by three revolutionary ideas:
- In 1925, Werner Heisenberg introduced matrix equations that removed the Newtonian elements of space and time from quantum mechanics;
- In 1926, Max Born proposed that quantum mechanics were best understood by probabilities;
- In 1927, Heisenberg had formulated his uncertainty principle defining the mathematical model to describe the fundamental limit of the precision with which certain pairs of physical properties of a particle (known as complementary variables) can be known.
Heisenberg’s first breakthrough idea was first proposed in his paper Über quantentheoretische Umdeutung kinematischer und mechanischer Beziehungen (“Quantum Theoretical Re-interpretation of Kinematic and Mechanical Relations”) which appeared in Zeitschrift für Physik in September 1925. Reportedly, Heisenberg in correspondence with Wolfgang Pauli had been working on the paper while recovering from hay fever. The purpose of the paper was to attempt to describe the energy levels of a one-dimensional anharmonic oscillator via observable parameters such as transition probabilities for quantum jumps (Segrè, 1980). The paper laid the groundwork for what is now known as matrix mechanics, which Heisenberg later developed in collaboration with Born and Pascual Jordan.

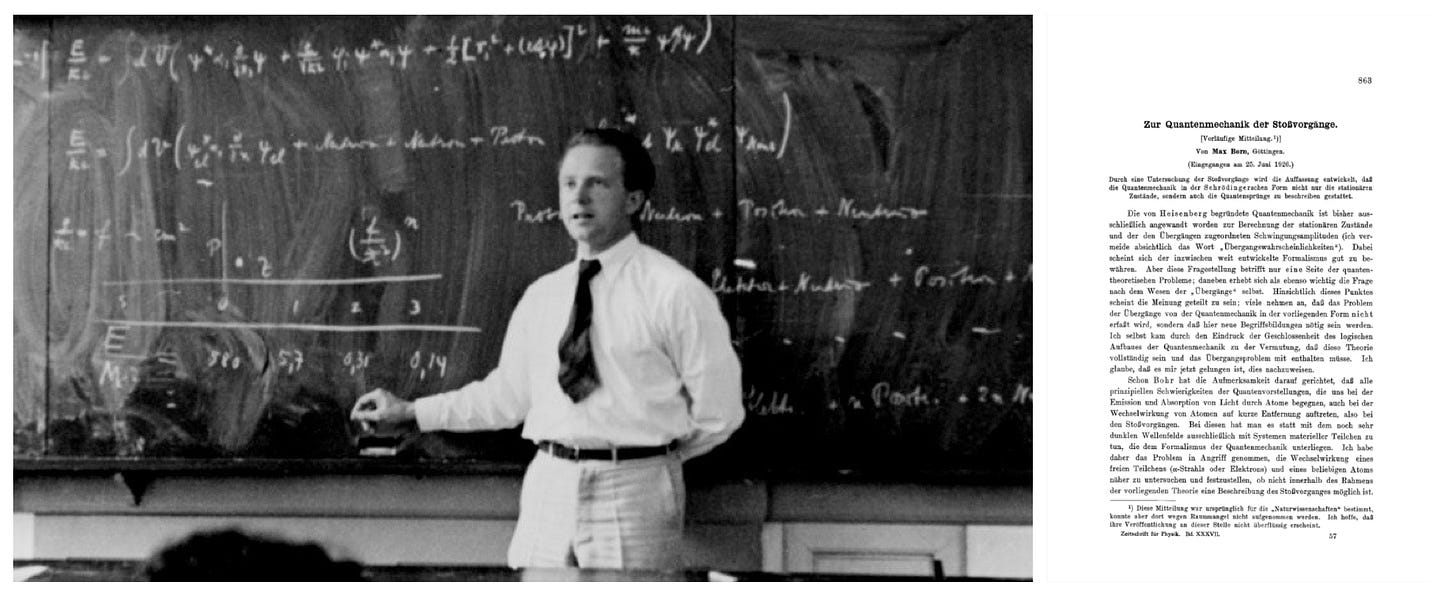
What is now known simply as the Born rule, giving the probability that a measurement on a quantum system will yield a given result was first introduced by Max Born in the 1926 paper Zur Quantenmechanik der Stoßvorgänge (“On the Quantum Mechanics of Collisions”). In the paper, Born solves the Schrödinger equation (postulated a year before) for a scattering problem. The rule is now considered a fundamental law of quantum mechanics, despite Einstein’s later famous criticism of its ultimate claim:
“I, in any case, am convinced that He does not play dice”. — Einstein
Heisenberg was by 1927 working as a lecturer in Bohr’s Institute for Theoretical Physics at the University of Copenhagen (now known simply as the Niels Bohr Institute). By February, Heisenberg already had his later paper Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik (“On the Descriptive Content of Quantum Kinematics and Mechanics”) where he introduces the Heisenberg uncertainty principle in draft form. He inquired to Bohr to have it forwarded to Einstein (American Institute of Physics, 1998), which Bohr did.
“Even in principle, we cannot know the present in all detail. For that reason everything observed is a selection from a plenitude of possibilities and a limitation on what is possible in the future…. The more precisely the position is determined, the less precisely the momentum is known in this instant, and vice versa.” — Werner Heisenberg (1927)
By the time of the conference in October, Born and Heisenberg were so confident in their three results that they proclaimed that quantum mechanics was “complete and irrevocable” (American Institute of Physics, 1998):
While we consider.. a quantum mechanical treatment of the electromagnetic field.. as not yet finished, we consider quantum mechanics to be a closed theory, whose fundamental physical and mathematical assumptions are no longer susceptible of any modification.. On the question of the 'validity of the law of causality' we have this opinion: as long as one takes into account only experiments that lie in the domain of our currently acquired physical and quantum mechanical experience, the assumption of indeterminism in principle, here taken as fundamental, agrees with experience.
- Born & Heisenberg (1927). "Quantum Mechanics". Proceedings of the Fifth Solvay Congress
Einstein, needless to say, did not agree.
“Quantum mechanics is very impressive. But an inner voice tells me that it is not yet the real thing. The theory produces a good deal but hardly brings us closer to the secret of the Old One” — Einstein
Einstein’s First Criticism
Einstein’s first serious criticism of the Copenhagen interpretation of quantum mechanics (and what is considered the start of what would become an ever-growing literature on the interpretation of the quantum principle) began during the Solvay Conference in 1927 (Mehra, 1975). Over dinner, during after-dinner discussions and at breakfast, Einstein debated Bohr and his followers on the question of whether quantum mechanics in its present form could be considered complete. Einstein illustrated his points with increasingly clever thought experiments intended to prove that position and momentum could in principle be simultaneously known to arbitrary precision. (Pais, 1982). One of the thought experiments Einstein proposed went as follows:
Thought Experiment: The Slit Experiment. Consider a particle passing through a slit of width d. The slit introduces uncertainty in momentum of approximately h/d because the particle passes through the wall. But, let us determine the momentum of the particle by measuring the recoil of the wall. In doing so, we find the momentum of the particle to arbitrary accuracy by conservation of momentum.
In other words, Einstein is proposing that if one assembles an experimental apparatus consisting of a wall with a narrow vertical slit and a beam of light pointed towards the wall, after having passed through the slit, the wave function of the light will diffract. Heisenberg’s uncertainty principle says that the uncertainty in momentum introduced by the slit is approximately h/d because the photon passes through the wall. By measuring the recoil of the wall, Einstein claims, one would find the momentum of the photon.

Bohr’s Response
Niels Bohr’s elegant response to Einstein’s proposed thought experiment was simple. He argued that the wall which the photon passes through is indeed a quantum mechanical system as well. As such, in order to measure the recoil of the wall to an accuracy of Δp, the momentum of the wall must also be known to this accuracy before the particle passes through. The implication is that at this degree of accuracy, the position of the wall is in fact uncertain as well, just as it is for the particle passing through it. As such, the position of the slit is equal to h/Δp and if the wall’s momentum is known precisely enough to measure the recoil, the slit’s position is uncertain enough to disallow a measurement of its position, in accordance with Heisenberg’s uncertainty principle.
By the following year (1928), the consensus in the physics community was that Einstein had lost the debate, and so that indeed, quantum mechanics as formulated by the Copenhagen school lead by Bohr, appeared to be complete (Isaacson, 2007). Even many of Einstein’s closest allies conceded, including French Nobel Laureate Louis de Broglie (physics, 1929).
Einstein’s Second Criticism
Einstein persevered. At the Sixth Solvay International Conference on magnetism (1930), he came armed with a new thought experiment. Again narrowing in on the problem of indeterminacy (i.e. lack of clear causation, loss of predictability) implied by the Copenhagen interpretation, Einstein proposed:
Thought Experiment: The Light Box. Consider a box containing electromagnetic radiation and a clock which controls the opening of a shutter which operated so quickly that it would allow only one photon to escape at a time. The box would first be weighed exactly. Then, at a precise moment, the shutter would open, allowing a photon to escape. The box would then be weighed again.

The key idea of the experiment is that Einstein’s darling, the then well-established relationship between mass and energy, E = mc², allows the energy of the particle to be determined (as we know the mass of a photon and the speed of light) (Pais, 1982).
Einstein’s new thought experiment reportedly baffled Bohr, who walked around the conference from participant to participant trying to convince them that Einstein’s thought experiment couldn’t be true.
"It was a real shock for Bohr...who, at first, could not think of a solution. For the entire evening he was extremely agitated, and he continued passing from one scientist to another, seeking to persuade them that it could not be the case, that it would have been the end of physics if Einstein were right; but he couldn't come up with any way to resolve the paradox. I will never forget the image of the two antagonists as they left the club: Einstein, with his tall and commanding figure, who walked tranquilly, with a mildly ironic smile, and Bohr who trotted along beside him, full of excitement." - Leon Rosenfeld
Bohr’s Triumph
After a sleepless night, Bohr worked out a response to Einstein, which is sometimes referred to as “Bohr’s Triumph” for the structure of its argument. Ironically, his solution depends on Einstein’s own theory of general relativity, as Bohr argued that after emitting a photon, the loss of weight causes the box to rise in the gravitational field (Pais, 2005):
The observer returns the box to its original height by adding mass until the weight points to its initial position. It takes a certain amount of time for the observer to perform this procedure. How long it takes depends on the strength of the spring and on how well-damped the system is. If undamped, the box will bounce up and down forever. If over-damped, the box will return to its original position sluggishly.
The longer that the observer allows the damped spring-mass system to settle, the closer the pointer will reach its equilibrium position. At some point, the observer will conclude that his setting of the pointer to its initial position is within an allowable tolerance. There will be some residual error Δq in returning the pointer to its initial position. Correspondingly, there will be some residual error Δm in the weight measurement.
Adding the weights imparts a momentum p to the box which can be measured with an accuracy of Δp delimited by ΔpΔq ≈ h. It is clear that Δp < gtΔm, where g is the gravitational constant. Viewed together, gtΔmΔq > h.
From Bohr’s reasoning above, Einstein’s general relativity informs us that while the box has been at a height different than its original height, the clock inside the box (necessary to ensuring that the shutter only emits one photon) has been ticking at a rate different than its original rate. The gravitational red shift formula tells us that there will be an uncertainty equal to Δt = c⁻²gtΔq in the determinacy of time at zero t₀, the moment of the emission of the photon. Hence,
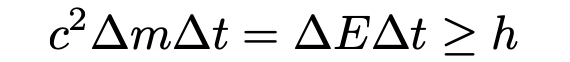
Conclusion: According to Heisenberg’s uncertainty principle, the accuracy with which the energy of the photon is measured restricts the precision with which its momentum of emission can be measured. Bohr showed that in order for Einstein’s thought experiment to work, his ticking light box would have to be suspended by a spring in the middle of a gravitational field.
Zing.
Einstein was reportedly gracious in his defeat. The following September, he nominated Heisenberg and Schrödinger for the Nobel Prize, stating, somewhat ambivalently,
“I am convinced that this theory undoubtedly contains a part of the ultimate truth.”
Einstein’s Third Criticism
Following the “defeat” of his former objections that the Copenhagen interpretation of quantum mechanics was complete, Einstein ceased attempting to search for inconsistencies in indeterminism, and instead focused on other aspects of quantum mechanics with which he disagreed. In essence, Einstein had accepted the fact that as a practical matter, it is impossible to simultaneously determine the value of certain incompatible quantities. The debate persisted, however, as to whether or not such quantities actually have precise values, even if they cannot be measured. Einstein continued to argue that indeed, even quantum probabilities are epistemic, not ontological in nature and so that the Copenhagen interpretation must still be incomplete, in that it claims the opposite.
“I have the greatest consideration for the goals which are pursued by the physicists of the latest generation which go under the name of quantum mechanics, and I believe that this theory represents a profound level of truth, but I also believe that the restriction to laws of a statistical nature will turn out to be transitory” — Einstein
Hidden Variable Theories
Einstein’s continued insistence on the epistemic nature of reality would spark lines of research into so-called hidden variable theories, the most famous being the so-called Bohm interpretation.
Worth noting here, as Max Jammer writes is that “Einstein never proposed a hidden variable theory.” Rather, he explored the possibility of a hidden variable theory, and wrote a paper describing his exploration, but withdrew it from publication because he felt it was faulty. The paper was entitled Bestimmt Schrödinger’s Wellenmechanik die Bewegung eines Systems vollständig oder nur im Sinne der Statistik? (“Does Schrödinger’s wave mechanics determine the motion of a system completely or only in the statistical sense?”) and was presented by Einstein at a meeting of the Academy of Sciences in 1927. The fault he found was possibly that contrary to his intention, the paper argued for the non-separability of entangled systems (Baggott, 2004), which Einstein reportedly regarded as absurd.
Einstein’s Fourth Criticism
Both Bohr and Einstein were subtle men. Einstein tried very hard to show that quantum mechanics was inconsistent; Bohr, however, was always able to counter his arguments. But in his final attack Einstein pointed to something so deep, so counterintuitive, so troubling, and yet so exciting, that at the beginning of the twenty-first century it has returned to fascinate theoretical physicists. Bohr’s only answer to Einstein’s last great discovery — the discovery of entanglement — was to ignore it. — Leonard Susskind
By 1934, Einstein had fled Europe to join the nascent Institute for Advanced Study, then housed in Princeton University’s Fine Hall. His first published work as a fellow there concerned what he found to be the continued unsatisfactoriness of the (by then) widely accepted Copenhagen interpretation of quantum mechanics. Known as the ‘EPR paradox’, it is now widely considered Einstein’s best argument against the completeness of quantum mechanics. Published in the 1935 paper Can Quantum-Mechanical Description of Physical Reality be Considered Complete?, the paper was co-written with Boris Podolsky and Nathan Rosen, and proposed another thought experiment (Isaacson, 2007):
Thought Experiment: Quantum Entanglement. Consider two particles that have collided or which have been created in such a way that they have properties which are correlated. The total wave function for the pair links the positions of the particles as well as their linear momenta. Observation of the position of the first particle allows us to determine precisely the position of the second particle no matter how far the pair have separated. Likewise, measuring the momentum of the first particle allows us to determine precisely the momentum of the second particle. "In accordance with our criterion for reality, in the first case we must consider the quantity P as being an element of reality, in the second case the quantity Q is an element of reality"
In the paper, Einstein, Podolsky and Rosen define physical reality as
If, without in any way disturbing a system, we can predict with certainty (i.e., with probability equal to unity) the value of a physical quantity, then there exists an element of physical reality corresponding to this physical quantity.
Einstein concluded that the second particle, which has never been directly observed, must at any moment have both a position and a momentum which is real, and that quantum mechanics does not account for these features of reality. From Heisenberg’s uncertainty principle, we know that it is impossible to measure both the position and the momentum of a particle simultaneously. However, even though their values can only be determined in distinct contexts of measurement, Einstein claimed that they must both have definite values. The only other alternative, Einstein claimed, would be that measuring e.g. the position of the first particle instantaneously affected the other (Fine, 2017)
“No reasonable definition of reality could be expected to permit this”
Einstein later summarized their argument as follows:
Consider a mechanical system consisting of two partial systems A and B which interact with each other only during a limited time.
Let the ψ function [i.e., wave function ] before their interaction be given. Then the Schrödinger equation will furnish the ψ function after the interaction has taken place.
Let us now determine the physical state of the partial system A as completely as possible by measurements. Then quantum mechanics allows us to determine the ψ function of the partial system B from the measurements made, and from the ψ function of the total system. This determination, however, gives a result which depends upon which of the physical quantities (observables) of A have been measured (for instance, coordinates or momenta).
Since there can be only one physical state of B after the interaction which cannot reasonably be considered to depend on the particular measurement we perform on the system A separated from B it may be concluded that the ψ function is not unambiguously coordinated to the physical state. This coordination of several ψ functions to the same physical state of system B shows again that the ψ function cannot be interpreted as a (complete) description of a physical state of a single system.
Excerpt, Albert Einstein (1936). "Physics and Reality". Journal of the Franklin Institute. pp. 221
Bohr’s Response
Bohr was reportedly stunned when he read the Einstein, Podolsky and Rosen paper, which took him six weeks to respond to, with a paper of exactly the same title: Can Quantum-Mechanical Description of Physical Reality be Considered Complete?, also published in Physical Review.
Prior to the EPR paper, Bohr had maintained that disturbance caused by the act of observation was the physical explanation for quantum uncertainty. After hearing the EPR thought experiment however, Bohr was forced to confront that “there is no question of a mechanical disturbance of the system under investigation.” On the other hand, he noted that the two particles are one system described by one quantum function. As such, in Bohr’s view, the EPR paper in no way dispelled Heisenberg’s uncertainty principle (Pais, 1982).
In the following years for the rest of their lives, the large majority of physicists continued to follow Bohr’s leadership, and Einstein was virtually shunned as he continued, in isolation, pursuing his (ultimately unrealized) unified field theory.
Epilogue
Despite their (at times) haughty debates, Bohr and Einstein were actually mutual admirers from beginning to end. They first met in the spring of 1920, in Berlin. By that point, both had already done the majority of the work they would later be renowned for. Einstein’s contributions to nascent quantum theory had launched a revolution, and his true masterpiece, general relativity, had finally been confirmed in the year before by the expedition of Arthur Eddington to observe the solar eclipse of the 29th of May 1919. Bohr’s model of the atom was already widely accepted and shown to agree mostly with experiment.
One does get the sense that Einstein and Bohr in some sense were long lost brothers. They had similar interests, were of similar age (Einstein was six years older than Bohr) and from similar places. By 1920, Einstein was a German citizen living in Berlin. Bohr was Danish, and had recently founded the Institute of Theoretical Physics at the University of Copenhagen, less than eight hours away by train. Indeed, following their first meeting, Einstein wrote to Bohr
“Not often in life has a human being caused me such joy by his mere presence as you did”
The feeling was clearly mutual, as Bohr later replied
“To meet you and to talk with you was one of the greatest experiences I ever had”
The two men would both receive the Nobel Prize in Physics in the next few years (Einstein in 1921, Bohr in 1922) and despite their later fierce debates, there was also plenty of scientific harmony between them (Pais, 1982). In 1922 for instance, Einstein wrote to Ehrenfest that
Letter from Einstein to Ehrenfest (1922)
At present, I am reading a major lecture by Bohr [presumably Bohr's contribution to the third Solvay Conference in 1921] which makes his world of thought wonderfully clear. He is truly a man of genius. It is fortunate to have someone like that. I have full confidence in his way of thinking.
Although they did not meet very often, or correspond very voluminously, their mutual admiration persisted, as Einstein’s secretary Helen Dukas later stated:
“They loved each other warmly and dearly”